
Scientists at Imperial College London are leading on the development and testing of the new method of stimulating the brain, which could provide an alternative treatment for brain diseases such as Alzheimer’s, and its associated memory loss.
Known as temporal interference (TI), the non-invasive method works by delivering electrical fields to the brain through electrodes placed on the patient’s scalp and head.
By targeting the overlapping electrical fields researchers were able to stimulate an area deep in the brain called the hippocampus, without affecting the surrounding areas—a procedure that until now required surgery to implant electrodes into the brain.
The approach has been successfully trialed with 20 healthy volunteers for the first time by a team at the UK Dementia Research Institute (UK DRI) at Imperial and the University of Surrey.
Their initial findings, published in the journal Nature Neuroscience, show that when healthy adults perform a memory task while receiving TI stimulation it helped to improve memory function.
The team is now conducting a clinical trial in people with early-stage Alzheimer’s disease, where they hope TI could be used to improve symptoms of memory loss.
Dr. Nir Grossman, from the Department of Brain Sciences at Imperial College London, who led the work said, “Until now, if we wanted to electrically stimulate structures deep inside the brain, we needed to surgically implant electrodes which of course carries risk for the patient, and can lead to complications.
“With our new technique we have shown for the first time, that it is possible to remotely stimulate specific regions deep within the human brain without the need for surgery. This opens up an entirely new avenue of treatment for brain diseases like Alzheimer’s which affect deep brain structures.”
TI was first described by the team at Imperial College London in 2017 and shown to work in principle in mice.
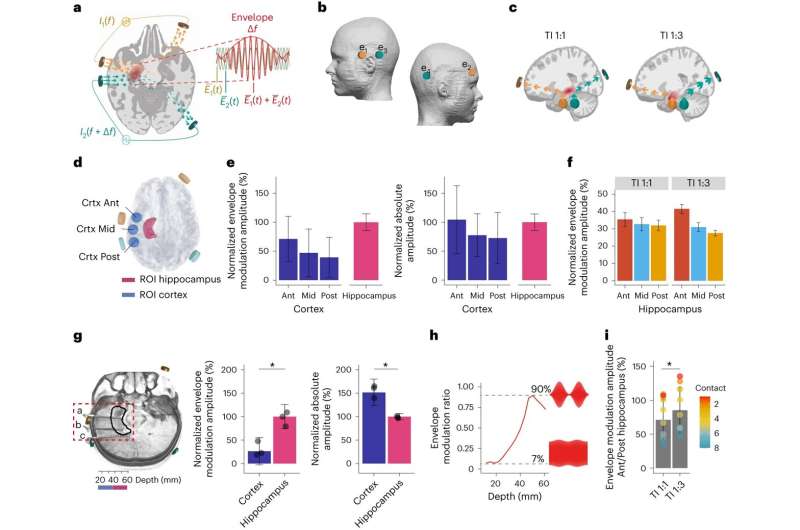
This latest work shows for the first time that TI is effective at stimulating regions deep within the human brain. According to the researchers, this could have broad applications and will enable scientists to stimulate different deep brain regions to discover more about their functional roles, accelerating the discovery of new therapeutic targets.
In the study, researchers first used post-mortem brain measurements to validate that the electric fields could be remotely focused on the hippocampus—a curved structure deep in the brain which plays a critical role in memory and learning.
The team then applied the TI stimulation to healthy volunteers while they were memorizing pairs of faces and names—a process heavily dependent on the hippocampus. Using functional magnetic resonance imaging (fMRI), the researchers showed that TI selectively affected the hippocampal activity evoked by the memory task.
Finally, the researchers repeated the procedure for a longer period of 30 minutes. This showed that TI stimulation during the task lead to improved memory accuracy.
Dr. Ines Violante, from the University of Surrey and Honorary Research Fellow at Imperial, and first author on the study, said, “The ability to selectively target deep brain areas using a non-invasive approach is very exciting as it provides a tool to investigate how the human brain operates and opens possibilities for clinical applications.”
“The combination of non-invasive imaging and brain stimulation will help us unravel the processes that support our cognitive functions, such as memory and learning. Knowledge of these processes and how they can be altered is essential to develop better individualized strategies to treat or delay the onset of diseases.”
Dr. Grossman added, “We hope this work will help to scale up the availability of deep brain stimulation therapies by drastically reducing cost and risk.”
“We are now testing whether repeated treatment with the stimulation over the course of a number of days could benefit people in the early stages of Alzheimer’s. We hope that this will restore normal brain activity in the affected areas, which could improve symptoms of memory impairment.”
This study is published simultaneously with a second paper led by researchers in École polytechnique fédérale de Lausanne (EPFL) in Switzerland, which independently validated the technology.
In the EPFL study, also published in Nature Neuroscience the researchers used the TI technology to focally stimulate a different deep brain area called the striatum and improve motor memory function in healthy volunteers.
More information:
Violante, I.R. et al. Non-invasive temporal interference electrical stimulation of the human hippocampus, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01456-8 www.nature.com/articles/s41593-023-01456-8
Wessel, M.J. et al. Noninvasive theta-burst stimulation of the human striatum enhances striatal activity and motor skill learning, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01457-7 www.nature.com/articles/s41593-023-01457-7
Journal information:
Nature Neuroscience
Source: Read Full Article