An underlying assumption of research on aging holds that dietary restriction (and drugs that mimic its effects) will slow aging to extend both lifespan and healthspan jointly. While eating a Spartan diet has been shown to robustly extend lifespan and delay age-related diseases in many species, a genome-wide analysis of 160 genetically distinct strains of the fruit fly D. melanogaster shows that lifespan and healthspan are not linked under dietary restriction. Results are published in Current Biology.
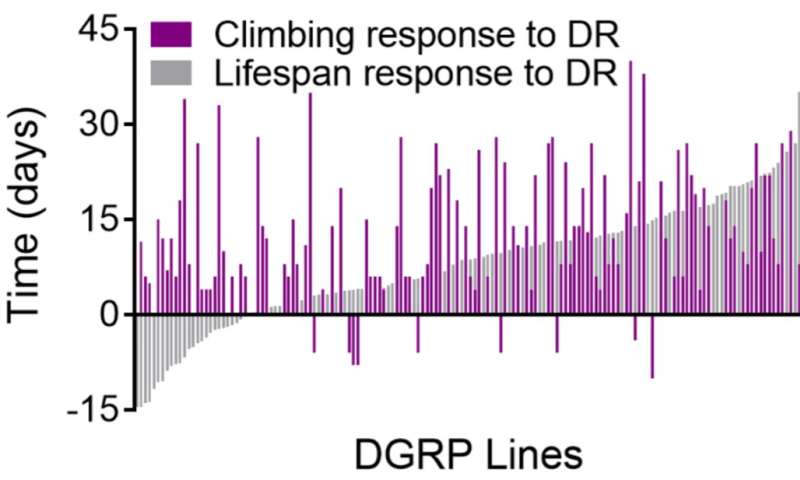
Though on average lifespan was extended and healthspan was increased, researchers from the Kapahi lab at the Buck Institute say the devil is in the details. In the study researchers measured nutrient-dependent changes in lifespan and tracked age-related changes in physical activity to measure healthspan. While 97 percent of strains showed some lifespan or healthspan extension in response to dietary restriction, only 50 percent of strains showed a significantly positive response to dietary restriction for both. Thirteen percent of the strains were more vigorous, yet died sooner with dietary restriction; 5 percent lived longer, but spent more time in poor health. The remaining 32 percent of the strains showed no benefits or detriments to lifespan or healthspan, or negative responses to both.
“Dietary restriction works, but may not be the panacea for those wanting to extend healthspan, delay age-related diseases, and extend lifespan,” said Pankaj Kapahi, Ph.D., Buck professor and senior author on the paper. “Our study is surprising and gives a glimpse into what’s likely going to happen in humans, because we’re all different and will likely respond differently to the effects of dietary restriction. Furthermore, our results question the idea that lifespan extension will always be accompanied by improvement of healthspan.”
Identifying (and sometimes naming) genes that make a difference
Utilizing genome-wide analysis researchers, led by postdoc Kenneth Wilson, Ph.D., found Drosophila genes that influenced healthspan or lifespan in a diet-dependent manner. They identified and named a fruit fly gene that regulates lifespan, decima, after the Roman god of fate; inhibiting this gene extended lifespan by reducing the production of insulin-like peptides, but did not improve age-related decline in climbing activity, which was used to track physical ability in the flies. They also named another gene daedalus, after the Greek mythological character Daedalus, who escaped prison with his son Icarus by crafting artificial wings (Icarus died because he flew to close to the sun). Inhibiting, daedulus delayed the age-related decline in physical activity upon dietary restriction, but only minimally influenced lifespan.
Wilson and his team tracked the lifespan and physical abilities of more than 50,000 flies during the course of the study. “It’s hard to ask and get relevant results in individuals,” he said. “With this method, we can ask questions in a much more robust manner and get answers at the population level.”
Implications for the field—lifespan should not be the gold standard
“Most experiments focused on lifespan and healthspan are done in a single strain of animal. Researchers usually do an intervention in one or a few backgrounds,” said Kapahi, adding that negative results are rarely reported, resulting in corroboration bias. “Our study takes an unbiased, systematic approach, to ask whether healthspan and lifespan traits are linked. We need to do more of this in the field.”
Kapahi also noted that climbing ability, used to track physical ability in the flies, is just one measure of healthspan. “Other traits associated with healthspan are also important to measure. We need to understand the genetics of age-related decline in other functions, such as vision and cognition. Working in simple animals, like the fruit fly, is a great place to do this efficiently. One lesson we have learned is that lifespan extension should not be the gold standard for determining the best means of dealing with age-associated maladies.”
What it means for consumers
“Lifespan-extending discoveries tend to get a lot of hype in the media as a way to cure all age-related maladies,” said Kapahi. “People need to be aware that their genetic background will likely have a major impact on how they will respond to an intervention. There is a need to develop markers in the field so we can predict how an individual will react to any particular intervention in terms of improving health and lifespan.”
Source: Read Full Article