Researchers in Italy have conducted a study showing that patients who had tested positive for infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) still had neutralizing antibodies against the virus 10 months later.
SARS-CoV-2 is the agent responsible for the coronavirus disease 2019 (COVID-19) pandemic that continues to pose a global public health threat and has now claimed the lives of more than 2.49 million people.
The researchers evaluated antibody responses over the course of 10 months among 30 people who were diagnosed with SARS-CoV-2 infection in the Umbria region of Italy between the 1st and 30th March 2020.
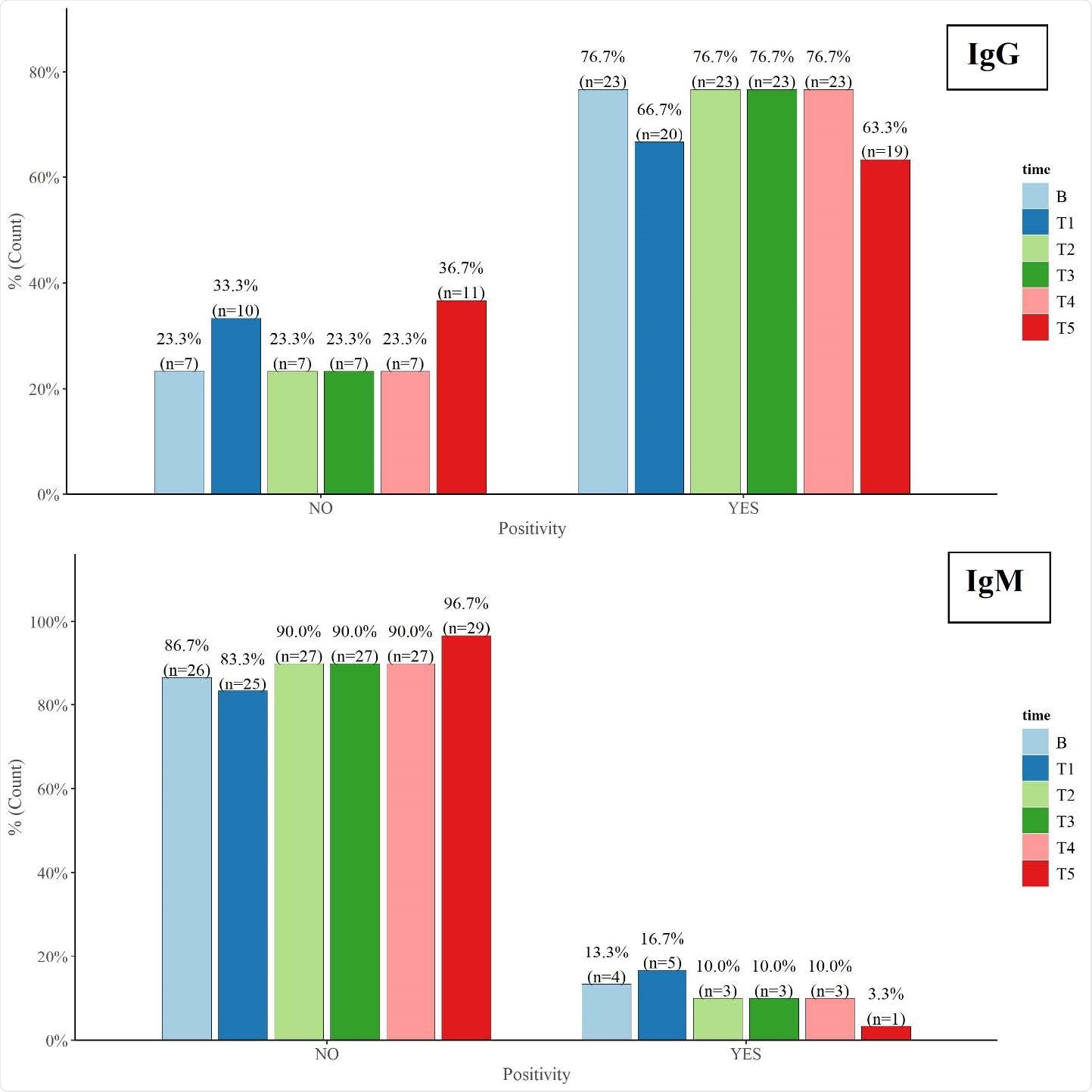
At ten months post-infection, 19 (63%) of the individuals still had detectable levels of neutralizing immunoglobulin G (IgG) antibodies against SARS-CoV-2.
Furthermore, no cases of reinfection occurred among any of the participants, despite a surge in daily infections with mutant strains occurring in the Umbria region during the study period.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.
Concerns surrounding antibody responses to SARS-CoV-2
Since the COVID-19 outbreak began in Wuhan, China, in late December 2019, studies have shown that humoral (antibody) responses among recovered patients can last anywhere from three months to more than eight months.
The SARS-CoV-2 virus expresses four key structural proteins, among which the spike (S) protein and nucleocapsid (N) protein are the most immunogenic.
The spike protein is the main structure the virus uses to bind to and gain entry to host cells.
As the initial stage of the infection process, the spike binds to the host cell receptor angiotensin-converting enzyme 2 (ACE-2) via its receptor-binding domain (RBD).
The spike RBD is the main target of SARS-CoV-2 neutralizing antibodies, which can be detected as soon as six days following confirmation of infection by polymerase chain reaction (PCR).
Studies published in 2020 have raised concerns regarding the duration of immunity provided by these neutralizing antibodies, warning that “rapidly waning immunity” could lead to false-negative immunoassay results.
“Improved understanding of immunity offered by the antibodies developed against SARS-CoV-2 is critical,” writes Puya Dehgani-Mobaraki from Associazione Naso Sano in Umbria, Italy and colleagues.
What did the researchers do?
The researchers conducted a longitudinal observational analysis of 114 patients in the Umbria region who had tested positive for SARS-CoV-2 by real-time quantitative PCR between the 1st and 30th March.
They conducted sequential serological tests over a ten-month period among 30 of the participants who attended all of the follow-up visits.
Blood samples were collected at six different time (T) points, with the first sample taken two months post-infection in the month of May (T0). Further samples were then taken one month (T1), three months (T2), five months (T3), six months (T4) and eight months (T5) after T0.
The presence and persistence of SARS-CoV-2-specific antibodies were assessed using two commercial chemiluminescence immunoassays (CLIA), with the CLIA positivity cut-off set at >1.01.
The participants were divided into those with mild disease (n=17) and those with a moderate-to-severe disease (n=13).
What did the study find?
The team reports that anti-spike-RBD IgG was detected in 19 (63.3%) of the 30 participants at T5 – ten months after infection was initially confirmed.
Among both the mild disease group and the moderate-to-severe disease group the trend of SAR-CoV-2-specifc IgM titers stayed below the CLIA positivity cut-off (>1.01) and significantly decreased over the ten-month period.
By contrast, the IgG titer trend stayed above the CLIA positivity cut-off in both groups and no significant changes in titer were observed.
Furthermore, “our study reported zero cases of reinfection despite the fact that the Umbria region currently is experiencing a surge in daily cases with mutant strains,” concludes the team.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Dehgani-Mobaraki P, et al. Neutralizing antibody responses 10 months after mild and moderately-severe SARS-CoV-2 infection. medRxiv, 2021. doi: https://doi.org/10.1101/2021.02.22.21252225, https://www.medrxiv.org/content/10.1101/2021.02.22.21252225v1
Posted in: Medical Research News | Disease/Infection News
Tags: Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, Blood, Cell, Coronavirus, Coronavirus Disease COVID-19, Enzyme, Immunoassay, Immunoassays, Immunoglobulin, Pandemic, Polymerase, Polymerase Chain Reaction, Protein, Public Health, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Serological Test, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Virus

Written by
Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Source: Read Full Article