The ability of the immune system to prevent the replication and subsequent transmission of pathogens is referred to as ‘sterilizing immunity.’ Primary infection with viral pathogens such as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can elicit sterilizing mucosal and systemic immunity, which is activated following secondary exposure to the pathogen at mucosal surfaces.
Vaccination mimics certain aspects of viral infection to train the immune system for future encounters with the actual pathogen. Aside from disease and mortality prevention, the ultimate purpose of a vaccination campaign is to create a strong sterilizing effect, lessen the carrier state, and break the transmission cycle in the population.
Study: Intramuscular mRNA BNT162b2 vaccine against SARS-CoV-2 induces robust neutralizing salivary IgA. Image Credit: ustas7777777 / Shutterstock.com
Background
The Pfizer/BioNTech BNT162b2 and Moderna mRNA-1273 coronavirus disease 2019 (COVID-19) vaccines have efficiently reduced the load of symptomatic COVID-19. However, the emergence of SARS-CoV-2 variants has raised concerns about the vaccine’s long-term protective capabilities. In these situations, 'sterilizing immunity' is critical for preventing the spread of SARS-CoV-2 and reducing the emergence of new variants.
The immunoglobulin gamma (IgG) responses to natural SARS-CoV-2 infection and their role against the spike protein and receptor-binding domain (RBD) in virus neutralization and disease prevention are well established. IgA is the most abundant Ig isotype in humans.
Pathogen-targeting IgA at mucosal surfaces is known to correlate with sterilizing immunity, thereby inhibiting the spread of respiratory and enteric viruses. However, the role of IgA in providing mucosal protection and systemic Ig responses has not been fully established.
Intramuscularly administered vaccines stimulate robust serum neutralizing antibodies, yet they are often less competent in eliciting sustainable ‘sterilizing immunity’ at the mucosal level. To this end, a new study published on the bioRxiv* preprint aims to determine the strong neutralizing mucosal component emanating from intramuscular administration of a messenger ribonucleic acid (mRNA) vaccine.
About the study
An independent enzyme-linked immunosorbent assay (ELISA) measurement of anti-RBD IgG and anti-RBD IgA in serum samples collected from pre-COVID (n=51), BNT162b2 vaccinee’s (n=17), and post-COVID-19 (n=22) convalescents was used to analyze humoral immune response and to detect transitory secretory dimeric IgA in the saliva of all vaccinees.
The potent neutralizing activity of the humoral component of mucosal defense, as well as its kinetic profile, were investigated. A methodology for quantitative comparison of immune reactivity and neutralization for humoral IgG and IgA responses in serum and saliva in molar equivalents was also developed.
A uniform approach for assessing antibody response that may be used universally, easing standardization in diagnostics and monitoring practices, decision-making in patient treatment, and comparative vaccine evaluations, was also submitted.
Study findings
Vaccinee saliva was found to contain transitory anti-RBD dimeric secretory IgA with strong neutralizing activity, as explained by its tetravalent nature. Polyvalent IgA was proposed to be a major mediator of powerful neutralizing activity in vaccinee saliva, and their levels did not alter after IgG depletion. Thus, reducing salivary IgA eliminated vaccine-induced neutralization.
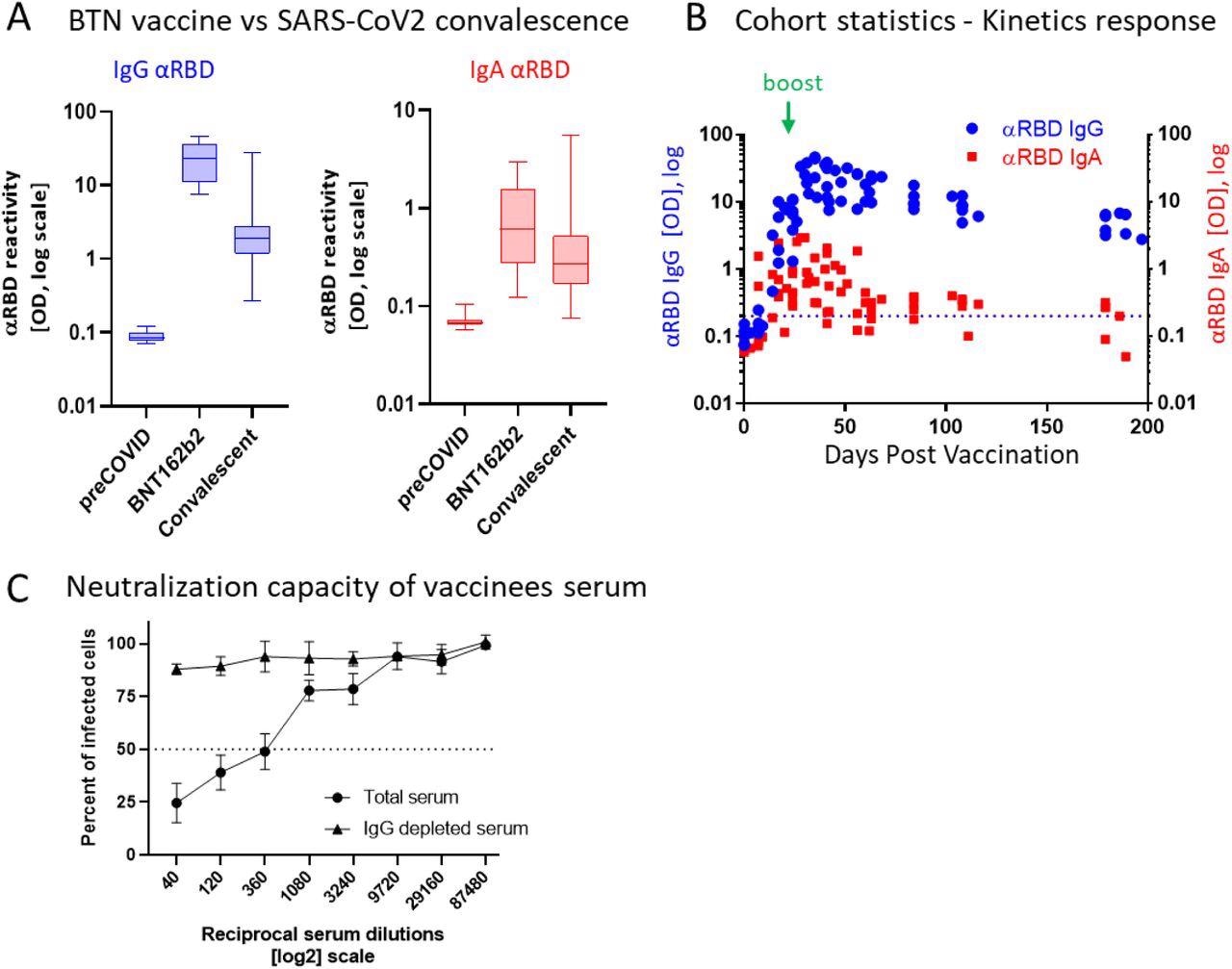
BNT162b2 vaccinees mount serum antiRBD-SARS-CoV-2, IgG and IgA with IgG showing strong neutralization potential. (A) Independent ELISA measurements of anti-RBD IgG and of anti-RBD IgA in serum samples collected from pre-COVID (N=51), BNT162b2 vaccinees (N=17) and post-COVID-19 (N=22) convalescents, as indicated. Convalescent samples were collected within two months post-recovery. BNT162b2 vaccinees samples represent the peaks of individual responses. (B) Quantitative kinetic profile of anti-RBD IgG (blue) and IgA (red) in serum sampled (N=76) in the vaccinees cohort (N=18), plotted as a function of days, post first vaccine dose. See Table S1 for cohort and sampling details. Independent ordinate axes for IgG (left, blue) and IgA (right, red) highlight the restricted, relative nature of the comparison between isotypes in this experiment, as discussed in the text, see also Figure 2 for subsequent developments. Green arrows indicate the timing of the second vaccine dose (the boost). (C) Serum neutralization assessed by SARS-CoV-2-Spike pseudotyped VSV-GFP-ΔG reporter assay on Vero-E6 cells. Neutralization is expressed as a percentage of pseudovirus-infected green cells without serum (total infection = 100%). Percentage of neutralization by sera of pool of four individual vaccinees (see the corresponding anti-RBD IgG and IgA values and times post-vaccination in Figure S1) are plotted as a function of the reciprocal values of sera dilutions displayed on a log2 scale, as indicated (filled circles, total serum, NT50 is reached on average at the dilution of ∼1:360, extrapolated by cross-section with the dashed line. The contribution of IgG to serum neutralization is evaluated by the depletion of the IgG isotype using anti-IgG specific magnetic beads (triangles). Results of three experimental repeats are represented.
Comparatively, in serum, IgG was the dominant neutralizing isotype, as demonstrated by its removal that resulted in a loss of neutralization. Surprisingly, and in contrast to the condition in saliva, remaining serum IgA lacked appreciable neutralizing activity, despite its substantially larger concentration that was approximately 30-fold higher in serum as compared to its levels in saliva.
The 50% neutralization capacity (NT50) molar measurements normalized to the binding reactivity values and greatly favored the stronger neutralization by salivary IgA. Anti-RBD IgA remained in the saliva for an extended duration after immunization, wherein it peaked two to four months and diminished only five to six months later, thus significantly outliving serum anti-RBD IgA.
The observation of interaction modes between surface SARS-CoV-2 lattice and mucosal dimeric IgA as compared to monomeric IgG and IgA in blood circulation indicated the importance of using lattice design to improve spatial surface-mimicry in next-generation subunit vaccines. To this end, mRNA-based vaccines allow host cells to display the natural configuration of SARS-CoV-2 spike membranous lattice upon its expression.
Significance
Overall, the results of the current study demonstrate that BNT162b2 vaccination causes a five-month transitory accumulation of anti-RBD IgA in the saliva, extending beyond the time range of detectable circulatory IgA. The researchers suggest that the polymeric origin of the salivary IgA molecules was responsible for the remarkably high specific neutralizing activity, thereby indicating that salivary anti-RBD IgA might represent a general nasopharyngeal humoral component of mucosal defense.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Stolovich-Rain, M., Kumari, S., Friedman, A., et al. (2022). Intramuscular mRNA BNT162b2 vaccine against SARS-CoV-2 induces robust neutralizing salivary IgA. bioRxiv. doi:10,1101/2022.02.17.480851. https://www.biorxiv.org/content/10.1101/2022.02.17.480851v1.full.
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Antibodies, Antibody, Assay, Blood, Coronavirus, Coronavirus Disease COVID-19, covid-19, Diagnostics, Enzyme, Immune Response, Immune System, immunity, Immunization, Immunoglobulin, Mortality, Nasopharyngeal, Pathogen, Protein, Receptor, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine, Virus

Written by
Snehal Jamalpure
Snehal Jamalpure is a postgraduate student in Microbial gene technology. Following completion of an M.Sc, she worked as a project fellow at the Council of Scientific and Industrial Research-Centre for Cellular and Molecular Biology in Hyderabad, India. Snehal has hands-on experience in protein-related and DNA-related experiments, cell culture, and handling of pathogenic strains ( Leishmania donovani ) in Biosafety level 3 facilities. Snehal is currently pursuing a Ph.D. in the subject of nanobioscience and is working at the interface of nanotechnology and biology.
Source: Read Full Article