Progress by a UMass Chan Medical School researcher in developing a gene therapy for a rare, crippling bone disease has reached an important milestone with demonstration of proof-of-concept in a humanized mouse model of fibrodysplasia ossificans progressiva (FOP) and human FOP patient-derived cells. The research is published in Nature Communications.
The work led by Jae-Hyuck Shim, Ph.D., associate professor of medicine in the Division of Rheumatology and a member of the Li Weibo Institute for Rare Diseases Research and the Horae Gene Therapy Center, is also featured in a fundraising campaign by the nonprofit International FOP Association (IFOPA).
FOP occurs in one per 1.36 million to 2 million people, according to Dr. Shim. This disease is characterized by abnormal bone formation in the skeletal muscle and in connective tissues. When there is any trauma to a muscle or other connective tissue, the resulting inflammation causes muscle to form like an extra skeleton that progressively locks up joints and causes immobility and severe pain. FOP typically strikes children. Shim said that average life span of FOP patients is 40.
Three years ago, Shim’s lab teamed up with the laboratories of Guangping Gao, Ph.D., the Penelope Booth Rockwell Professor in Biomedical Research, professor of microbiology & physiological systems, director of the Horae Gene Therapy Center and co-director of the Li Weibo Institute for Rare Diseases Research, and Jun Xie, Ph.D., associate professor of microbiology & physiological systems, to develop adeno-associated virus (AAV)-mediated gene therapy for FOP.
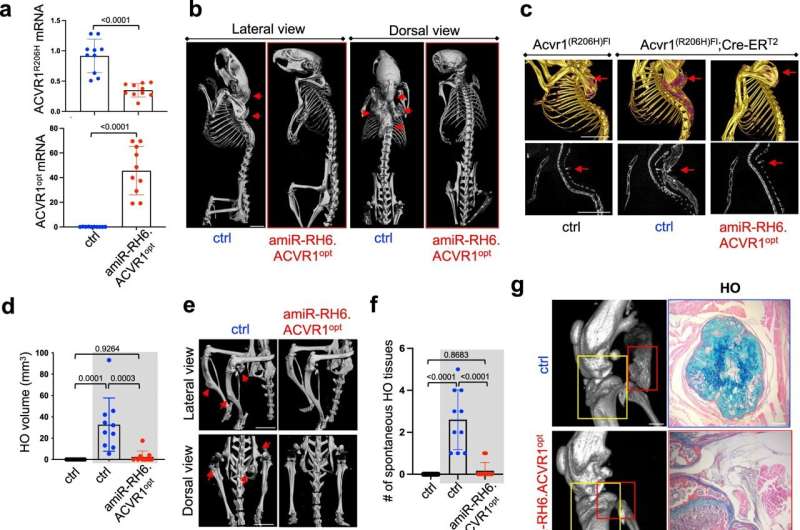
Their work has now demonstrated that AAV gene therapy is a potent suppressor of both traumatic and spontaneous extra-skeletal growth in FOP mice, which Shim hopes can be translated into clinical treatment in humans.
Dr. Gao said, “This is groundbreaking. So far, there is no treatment for FOP, and our paper really proved the concept. This could potentially be an effective approach to tackle this very difficult disease.”
“Skeletal gene therapy has been relatively underdeveloped mainly due to lack of a bone-specific carrier that can deliver therapeutic gene(s) to bone cells, compared to the whole gene therapy field,” continued Gao. “Dr. Shim and I have developed a new bone-specific AAV vector and using this vector, we have really made a big leap to prove a concept for preclinical viability of gene therapy for human skeletal diseases.”
Shim recalled that when he came to UMass Chan in 2016, there was no skeletal gene therapy system available. In collaboration with Gao, he found he could make more bone by removing a bone-forming suppressor gene or a bone-destroying gene via a bone-targeting AAV-mediated delivery, which could treat osteoporosis, bone fracture and inflammatory arthritis-induced bone loss.
So, with this idea, he also explored approaching two new rare skeletal diseases, FOP and osteogenesis imperfecta (OI), also known as brittle bone disease, from a complementary angle. The goal was to remove the ACVR1 mutant gene that causes extra-skeletal growth in FOP and replace it with a normal ACVR1. For the treatment of OI, the COL1A1 or 2 mutant gene is replaced with a normal COL1A1 or 2. Alternatively, a mutation in the ACVR1 or COL1A1 or 2 can be corrected by the gene editing machinery.
Shim and Gao licensed their AAV gene therapy for FOP to a company they cofounded, AAVAA Therapeutics, which along with the nonprofit IFOPA, is supporting further work in this field.
IFOPA spent time this summer on campus filming the laboratories of Shim, Gao and Anastasia Khvorova, Ph.D., the Remondi Family Chair in Biomedical Research and professor of RNA therapeutics for a video shown at the organization’s “In Pursuit of a Cure” fundraising campaign launch in September.
Source: Read Full Article