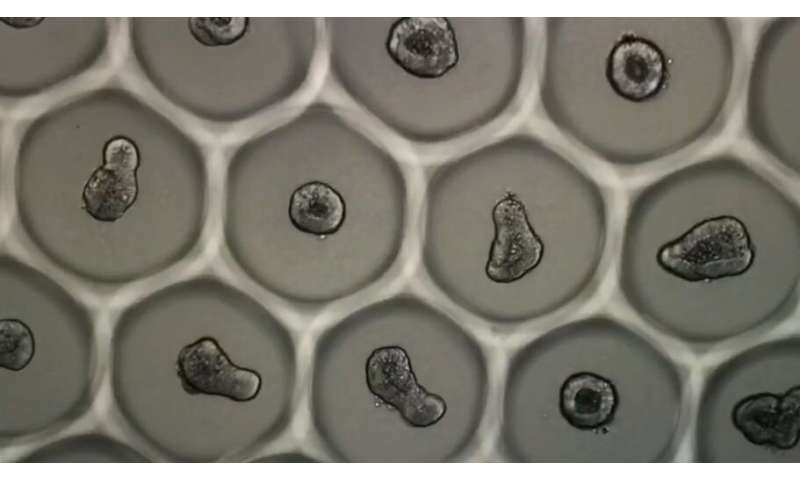
A recent innovation from an EPFL laboratory will enable, for the first time, mass production of standardized organoids. This breakthrough was achieved thanks to a customized guidance system that ensures homogenous cell culturing. Described in an article published today in Nature Biomedical Engineering, the technique paves the way for industrial uses, such as screening new drugs.
Over the past decade, organoid research has raised hopes, particularly in terms of personalized medicine and improved screening for new drugs. The structure and properties of these three-dimensional tissue cultures are similar to those of entire organs, creating unparalleled opportunities. However, widespread use of organoids has been hampered due to the cells’ disorderly growth. The ability to compare thousands of samples for industrial use requires that a series of identical “mini-organs” be created to enable automated monitoring. Thanks to the microstructured hydrogels it has developed, the Laboratory of Stem Cell Bioengineering at EPFL has made this a reality. The hydrogels act as tiny molds in which stem cells can propagate and differentiate uniformly. The new system—detailed in an article published today in Nature Biomedical Engineering—was successfully used in a study at Lausanne University Hospital (CHUV) that tested drug molecules on colorectal cancer.
Automated monitoring
Several years of research were needed to create the ideal environment for fostering controlled organoid growth. “We knew that one of the essential parameters was the rate at which stem cells aggregate,” says Nathalie Brandenberg, one of the article’s authors. The hydrogels are imprinted with holes only a few micrometers in diameter, creating a series of U-shaped microwells. Each well receives about 100 cells, which cluster together and form a relatively compact colony in about 30 minutes. The aggregated intestinal cells then grow and differentiate, producing functional intestinal organoids some 60 hours later. “We obtained a 92% success rate with these cultures,” Brandenberg says. The soft lithography technique is used to mass-produce the gels. The method seems simple but, in the words of Matthias Lütolf, the laboratory’s director, “developing a highly reliable design and manufacturing process for the gels, as well as methods for culturing the organoids in them, presented real scientific challenges.”
Suitable for various types of organoids
The concept was recently deployed at the CHUV and the Ludwig Institute for Cancer Research as part of anti-cancer drug testing. Thousands of tiny samples in neat rows quickly allowed researchers to generate and compare a large number of images and accurate real-time data on anatomical, physiological and even molecular aspects. The team also discovered that by selecting a specific diameter and density, the mini-organs they studied remained alive for sixteen days, enabling them to investigate the impact of active substances over time. Cell growth was not disturbed by daily additions of liquid or gel: fewer than 1% of the samples were negatively affected.
Since the system can be adapted as needed, its use is not restricted to screening anti-cancer drugs. “The diameter, depth and spacing between the microwells can be varied, as can the gel’s formulation. This means that different types and sizes of tissue can be cultured,” says Lütolf. The technology is currently being used in a pilot clinical study in collaboration with the CHUV to grow intestinal organoids using stem cells taken from patients with cystic fibrosis. The goal is to determine whether this new method can be used to find a specific drug combination for each patient. The initial results are encouraging.
A startup is born
Source: Read Full Article