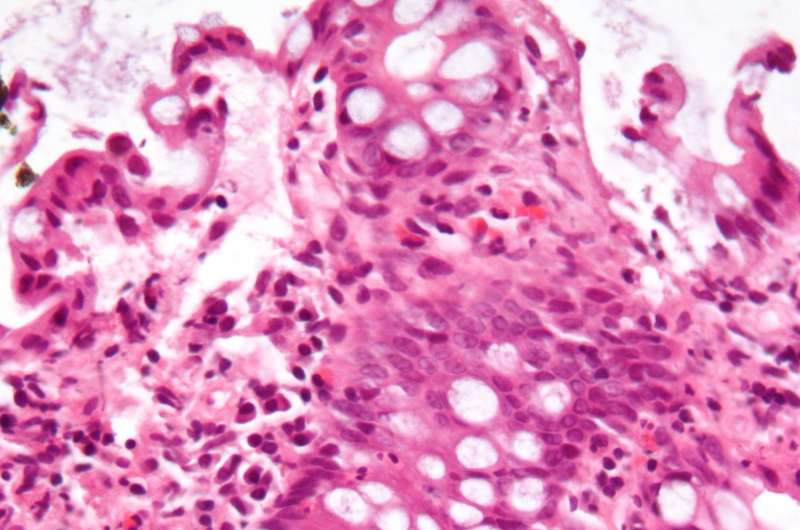
Intestinal fibrosis is a common feature of inflammatory bowel disease (IBD) and the primary cause of end-stage organ failure. Traditionally considered a bystander of inflammation, with negligible involvement in disease pathogenesis, new research published in Gastroenterology now shows that fibrosis has a direct bearing on disease progression in IBD.
The investigation was spearheaded by Nima Saeidi, Ph.D., Associate Professor of Surgery at the Massachusetts General Hospital (MGH) and Harvard Medical School, along with co-first authors, Shijie He, Ph.D., and Peng Lei, Ph.D.
The critical question posed by the investigators was how tissue stiffening influences the growth and differentiation of intestinal stem cells, which fuel the regeneration of intestinal epithelium?
This was addressed by developing a new in vitro platform, which allowed intestinal organoids to be cultured on an open lumen, planar system that could be manipulated experimentally.
The platform permitted the use of soft yet “tunable” substrates with biophysical properties mimicking native tissue, facilitating the long-term growth and differentiation of intestinal stem cells, similar to native epithelium.
Saiedi and colleagues discovered that upon elevating substrate stiffness to a similar range observed in IBD patients, both the number and capacity of stem cells to maintain homeostasis and cellular composition of the epithelium were potently reduced.
Concomitantly, the stem cells preferentially differentiated into goblet cells, leading to epithelial deterioration. Similar phenotypes were also noted in mouse models of IBD as well as in samples from human patients.
The investigators concluded that interfering with the molecular machinery involved in the cellular sensing of stiffness conferred protection against the detrimental effects of fibrosis and stiffening.
“These findings demonstrate that intestinal fibrosis and stiffening are critical components of IBD pathogenesisand that targeting mechanosensing and mechanotransduction pathways may offer an attractive therapeutic strategy for IBD,” says Saeidi.
The scientists also observed that despite the significant reduction in a specific population of stem cells, stiffening led to the expansion of another stem cell marker (OLFM4) outside the stem cell zone.
“Our observations that stiffening increased the expression of OLFM4 may have significant implications for the development of colitis-associated colorectal cancer,” says Dr. He.
A collaborative work between scientists from the Massachusetts General Hospital, MIT, Boston Children’s Hospital, Harvard T.H. Chan School of Public Health, and Boston University, additional MGH co-authors included Dr. Richard Hodin and Dr. Ruslan Sadreyev.
More information:
Shijie He et al, Stiffness Restricts the Stemness of the Intestinal Stem Cells and Skews Their Differentiation Towards Goblet Cells, Gastroenterology (2023). DOI: 10.1053/j.gastro.2023.02.030
Journal information:
Gastroenterology
Source: Read Full Article