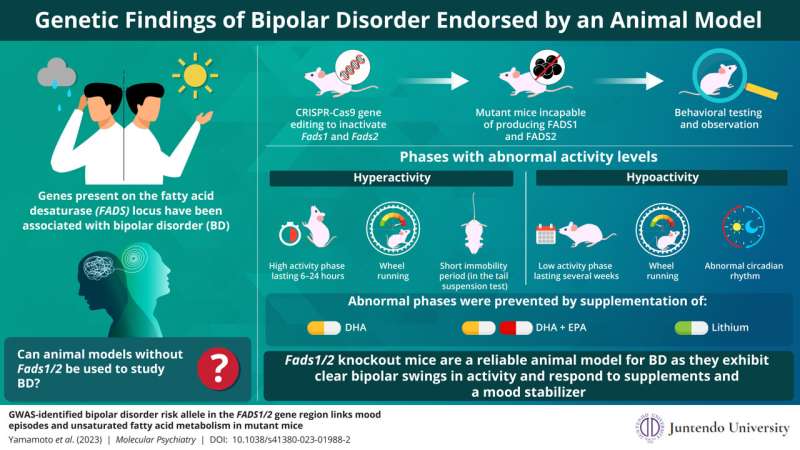
Bipolar disorder (BD) is a debilitating condition characterized by alternating states of depression (known as depressive episodes) and abnormal excitement or irritability (known as manic episodes). Large-scale genome-wide association studies (GWASs) have revealed that variations in the genes present on the fatty acid desaturase (FADS) locus are linked to an increased risk of BD.
Enzymes coded by FADS genes—FADS1 and FADS2—convert or “biosynthesize” omega-3 fatty acids into the different forms required by the human body. Omega-3 fatty acids like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are crucial for the brain to function, and a reduction in the synthesizing activity of these molecules seems to increase susceptibility to bipolar mood swings.
Research on most diseases involves establishment of an animal model of the disease. So, keeping this knowledge in mind, a team of researchers including Dr. Takaoki Kasahara and Hirona Yamamoto from RIKEN Brain Science Institute and Dr. Tadafumi Kato from Juntendo University in Japan, used CRISPR-Cas9 gene editing to create mutant mice that lack both Fads1 and Fads2 genes.
They then tested these models to see if they could serve as an animal model for BD. Their tests involved behavioral analyses and systematic observation of these mice over six months, to identify behavioral changes caused by the mutation. Their findings are detailed in a study published in Molecular Psychiatry.
The mice showed bipolar swings in behavior, featuring phases of abnormally low activity and phases of hyperactivity. Dr. Kato tells us, “The hyperactivity episodes, in which activity was far above the norm, usually lasted half a day.” During these episodes, the mice spent an increased amount of time running on wheels and were considerably less likely to remain still during tail suspension tests. Immobility during tail suspension tests is a widely used measure for depression-like behavior in mice.
Phases of low activity or “hypoactivity,” in which the mice exhibited low physical activity and spent an abnormally short period of time running on wheels, lasted much longer in the mutants—usually several weeks.
“Unlike other locomotor activities, wheel running in mice is a strongly goal-directed behavior having a significant reward value; thus, a reduction in wheel running is associated with markedly diminished pleasure—anhedonia—a core symptom of a depressive episode,” Dr. Kato explains. Mutant mice also exhibited abnormal circadian rhythms during hypoactivity episodes. Circadian rhythm is the cyclical pattern of physiological changes that normally coincides with daytime and night-time; it affects our sleep pattern.
To ascertain whether behavioral changes can be reversed by increasing the levels of omega-3 fatty acids, the researchers fed mutant mice supplements containing DHA or a combination of DHA and EPA. The occurrence of periods with abnormally low activity in mice reduced considerably when EPA and DHA were supplemented.
Administration of lithium, a mood stabilizer that is widely used to treat manic and depressive episodes in bipolar patients, had a similar effect on mutant mice. All of these indicate how similar BD in these mice models was to BD seen in humans.
Further, the researchers observed mice that lacked FADS enzymes exclusively in the brain to check if their behavioral changes can be attributed to molecular changes in brain cells. However, these mice failed to show bipolar swings in activity similar to mice that lacked FADS completely.
The study’s findings suggest that mutant mice lacking Fads1/2 may be helpful as an animal model to study the molecular roots of BD and the development and regulation of mood swings seen in the disorder. Moreover, since these mice respond positively to omega-3 fatty acid supplements and a mood stabilizer, they may help develop new treatments for BD.
More information:
Hirona Yamamoto et al, GWAS-identified bipolar disorder risk allele in the FADS1/2 gene region links mood episodes and unsaturated fatty acid metabolism in mutant mice, Molecular Psychiatry (2023). DOI: 10.1038/s41380-023-01988-2
Journal information:
Molecular Psychiatry
Source: Read Full Article