Researchers in the United States have shown that targeting the N-terminal domain (NTD) of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral spike protein could achieve broad neutralization of variants of concern.
The SARS-CoV-2 virus is the agent responsible for the ongoing coronavirus disease 2019 (COVID-19) pandemic, and the spike protein is the main structure the virus uses to bind to and infect host cells. This spike is the primary target of neutralizing antibodies following SARS-CoV-2 infection and, therefore, the focus of most vaccine design approaches.
The team – from Icahn School of Medicine at Mount Sinai in New York and Washington University School of Medicine in Missouri – says the findings could inform the design of next-generation vaccines that will protect against both currently circulating and newly emerging SARS-CoV-2 variants of concern.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
.jpg)
Researchers are trying to better understand vaccine-induced immune responses
As safe and effective COVID-19 vaccines are developed and rolled out in record time, researchers are urgently trying to better understand vaccine-induced immune responses, the broadly neutralizing epitopes that are targeted, and the effectiveness of these epitopes against newly emerging and potentially more transmissible viral variants.
Understanding the immunodominance of the spike protein at a structural level would help to identify the requirements for broader SARS-CoV-2 antibody responses and inform the development of next-generation vaccines.
The viral spike glycoprotein mediates the infection process when its receptor-binding domain (RBD) attaches to the host cell receptor angiotensin-converting enzyme 2 (ACE2).
Antibody responses to the spike protein in serum, the memory B cell compartment, and mucosal surfaces, have already been well characterized in terms of kinetics, binding specificity and neutralization potency.
“Anti-SARS-CoV-2 spike serum antibody titers after natural infection are variable, may decline to some degree over time and have suboptimal neutralization activity against more recent viral variants, despite being protective,” writes Goran Bajic and colleagues.
What did the researchers do?
Antibodies derived from memory B cells target both unique and overlapping epitopes that contribute to polyclonal epitopic coverage of the spike protein and preserve binding to viral variants of concern.
Bajic and colleagues investigated the early events involved in B cell activation following COVID-19 vaccination to structurally profile novel antibody epitopes.
The team had previously isolated and characterized plasmablast-derived antibodies from recipients of the Pfizer-BioNTech BNT162b231 vaccine.
The researchers observed that the overall neutralizing antibody responses were directed not only towards the spike RBD but also the NTD, suggesting co-dominance of these two domains.
“Since the NTD emerges as an important component of the vaccine-induced responses, we wanted to expand on our understanding of neutralizing epitopes in this region of the SARS-CoV-2 spike, for which only limited structural information is currently available,” writes Bajic and colleagues.
The researchers, therefore, focused on a vaccinee who had mounted a strong neutralizing antibody response to the spike NTD.
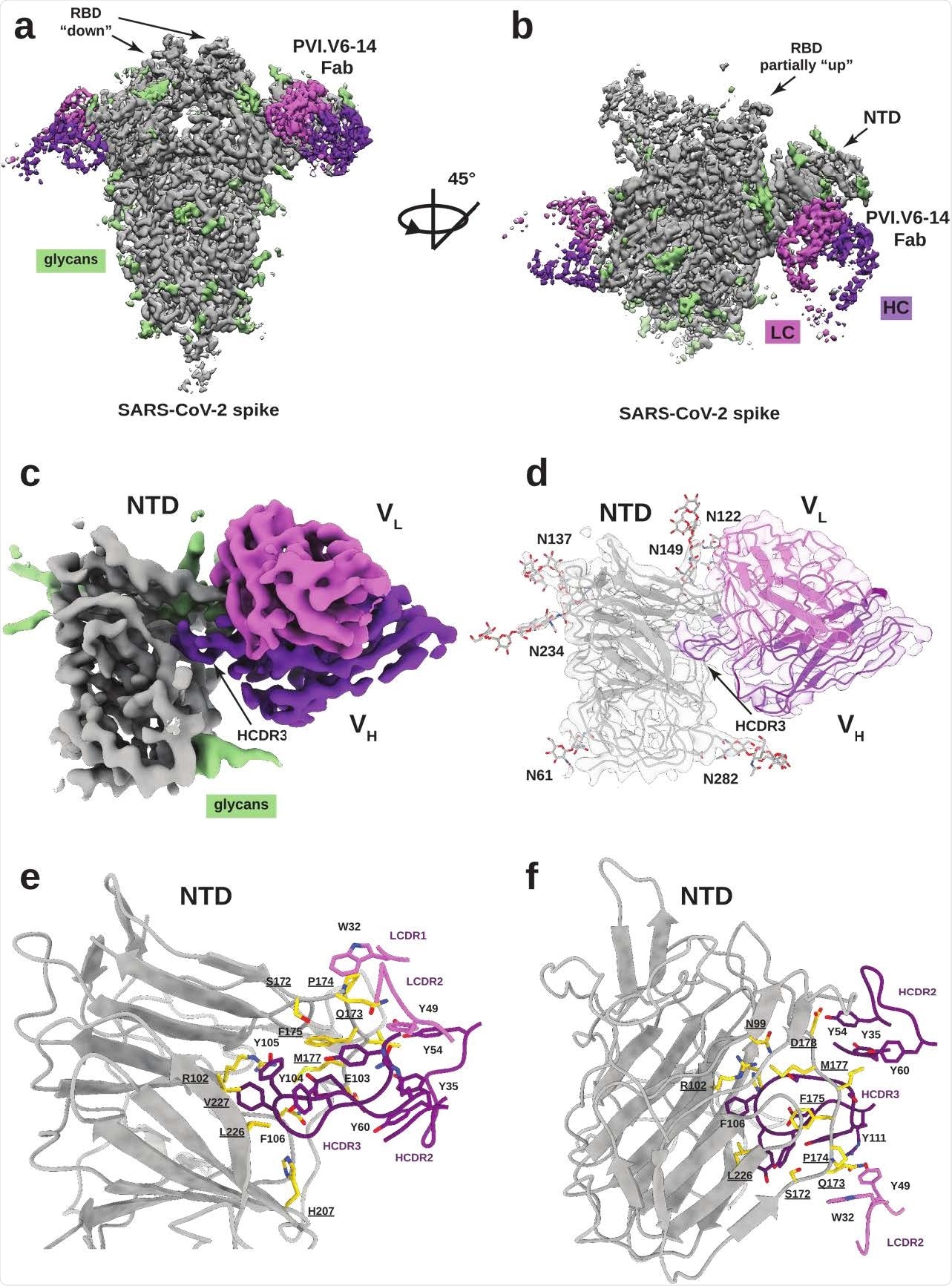
Analysis of that participant’s plasmablast response led to the identification of a neutralizing monoclonal antibody (mAb) called PVI.V6-14 that had heavy and light chains with no somatic hypermutation.
What did the current study find?
Now, using single-particle electron cryomicroscopy, the researchers have determined the high-resolution structure of this mAb in complex with the SARS-CoV-2 spike protein.
This revealed that PVI.V6-14 targets a previously uncharacterized neutralizing epitope on the side of the NTD.
The team found that PVI.V6-14 stabilized the NTD by inserting its heavy complementarity-determining region 3 (HCDR3) loop into a hydrophobic pocket that has previously been shown to bind the heme metabolite biliverdin.
“We found that mAb PVI.V6-14 belongs to an as of yet undescribed class of antibodies that bind within a hydrophobic cavity previously identified to bind biliverdin,” say the researchers.
Functional binding and neutralization data showed that PVI.V6-14 directly competes with biliverdin. In addition, due to the conserved nature of the epitope, the mAb maintains binding to the B.1.1.7 (alpha) and B.1.351 (beta) SARS-CoV-2 variants of concern.
Findings may pave the way for combined antibody therapies and next-generation vaccines
The team says the study supports the development of a combined antibody cocktail that targets both the RBD and the NTD.
“Our study illustrates the feasibility of targeting the NTD to achieve broad neutralization against SARS-CoV-2 variants,” writes Bajic and colleagues.
“Targeting the NTD offers an alternative to RBD-centric vaccine immunogen design and paves the way to next-generation vaccines that target NTD in addition to RBD and potentially to antibody therapeutics that combine both RBD- and NTD- targeting neutralizing mAbs,” they conclude.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Bajic et al. Structure of a germline-like human antibody defines a neutralizing epitope on the SARS-CoV-2 spike NTD. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.07.08.451649, https://www.biorxiv.org/content/10.1101/2021.07.08.451649v1
Posted in: Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, Cell, Coronavirus, Coronavirus Disease COVID-19, Electron, Enzyme, Germline, Glycans, Glycoprotein, Medicine, Metabolite, Monoclonal Antibody, Pandemic, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Therapeutics, Vaccine, Virus

Written by
Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Source: Read Full Article