The COVID-19 pandemic continues, but there is another global infectious disease that has a huge impact on the world. Tuberculosis (TB) causes over 1.5 million deaths and over 10 million infections per year. Estimates suggest around 10 years of progress in reducing the global burden of TB have been lost due to the COVID-19 pandemic, with 1.4 million fewer patients diagnosed and treated in 2020 compared with 2019.
Like many bacterial infections we treat with antibiotics, over time, mutations in the bacterial genome can give Mycobacterium tuberculosis (M. tuberculosis) the ability to survive treatment—thus becoming drug resistant. This can even happen in a single patient during the course of therapy. In recent years, this problem has been growing, and around half a million people suffer multi-drug resistant (MDR) TB every year.
This disease has a terrible impact on patients; the illness is severe, the treatment is long, and the drugs have brutal side-effects. Treatment for MDR-TB is also dramatically more expensive, and the cost to properly treat all cases rises into billions.
As part of EMBL’s Infection Biology research theme, researchers in my group in my group at EMBL-EBI have been working to improve therapy choice and surveillance through the sequencing of the pathogen genome. Below are some examples of how we are doing this.
Working with the WHO
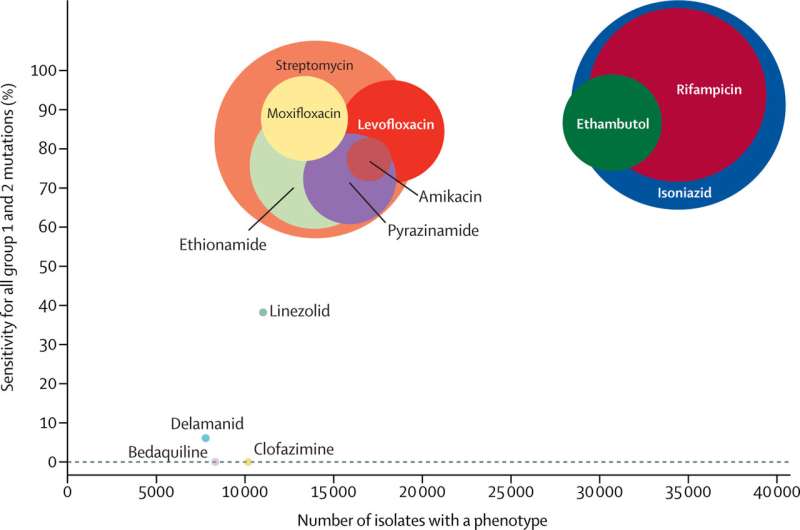
The rise of drug resistance has led to changes in the standard method for treating TB. The gold standard is to take a sample—usually sputum, the thick mucus made in the lungs—from a patient, isolate the M. tuberculosis, culture it for several weeks, and test which drugs the infection is susceptible to. This gives a personalized readout to see which drugs will work for this particular patient. This is really powerful information, but it can take up to two or three months, as M. tuberculosis is a slow-growing bacteria. In recent years, many studies have shown that mutations in the bacterial genome can be used to spot drug resistance; specific mutations confer resistance to specific drugs, and we are gradually accumulating information on the key mutations.
Unlike in the case of other bacterial diseases, the World Health Organisation (WHO) provides advice on what TB treatment to administer depending on which drugs will or won’t work on a particular infection. However, they have not given any recommendations on determining which mutations affect the efficacy of specific drugs. My team is part of a technical working group for WHO and we have analyzed over 40,000 M. tuberculosis genomes and cataloged the mutations present. Combining this information with a wide range of other literature, including the enormous genotype and phenotype dataset of the CRyPTIC project, the working group has just published a knowledge base of all these high-confidence mutations.
Implementing sequencing in Argentina’s healthcare system
Thanks to a UKRI Global Challenges Research Fund (GCRF), my team has just spent two years working with colleagues at the Malbran Institute in Argentina, establishing sequencing analysis workflows for TB. As part of this work, we wanted to understand how to make routine sequencing work within the Argentine health infrastructure, and enable appropriate reporting. This collaboration contributed to a successful case made to the Argentine ministry and has led to the adoption of routine mycobacterial sequencing across Argentina.
“It has been challenging moving this project forward during the SARS-CoV-2 pandemic, but it was important to us to establish and validate our analysis pipelines and operating procedures,” said Josefina Campos, Coordinator of Genomics and Bioinformatic Platforms at Administración Nacional de Laboratorios e Institutos de Salud (INEI-ANLIS), Argentina. “Although Argentina is not a high prevalence country, there are regions where TB is a real concern. This collaboration with EMBL-EBI has been very fruitful, and it contributed to our national decision-making, to implement routine sequencing for TB.”
Nanopore technology validation
The end goal of sequencing-based diagnostics for TB is a same-day test that returns a “resistance profile” informing the choice of therapeutic regimen, while the patient is still in the clinic. There are two components needed for this to work: 1) a way to avoid culturing the bacteria for two weeks, and 2) a rapid turnaround sequencing process in point of care settings. The first one is addressed by sequencing sputum from the patient directly, without culture. The second can be addressed by using a handheld Oxford Nanopore MinION sequencer.
My Ph.D. student Michael Hall recently published a preprint in MedRxiv, working with collaborators in South Africa, Madagascar, and England, evaluating nanopore technology as an alternative to other established methods for standard sequencing of M. tuberculosis. The two things they wanted to check were: 1) does this method give the same drug resistance readout when compared with other established methods, and 2) does it detect the same “outbreak clusters” which public health teams use for TB track-and-trace. They found they were able to obtain almost identical drug resistance predictions compared to other established sequencing methods and could easily identify patients from different outbreak clusters. The latter fact was particularly important for the co-authors in Madagascar, who wanted to use this information to carefully drive contact tracing across the country.
“It is a great pleasure to collaborate with EMBL-EBI. This project is very important to our work in Madagascar, where we have built infrastructure and expertise for nanopore sequencing,” said Simon Grandjean Lapierre, Associate Professor in the Microbiology, Infectious Diseases and Immunology Department of Université de Montréal and visiting researcher at Institut Pasteur de Madagascar. “It was critical for us to validate how we could use this technology for outbreak investigations.”
Clinical trials in Madagascar
My team will also soon collaborate with researchers from Institut Pasteur de Madagascar and Université de Montréal for a cluster randomized controlled trial, in which pre-existing groups of individuals will be randomly allocated to distinct Tuberculosis control intervention strategies. This will help assess the added value of using genomic information to guide track-and-trace in high-TB-incidence villages in rural Madagascar. The aim is to assess whether and how systematic M. tuberculosis whole-genome sequencing can support TB case finding in high-incidence rural settings.
“We are very excited to be collaborating with Université de Montréal and EMBL-EBI to start measuring the potential impact of sequencing TB on case detection in a population that would need it the most, like in Madagascar,” said Niaina Rakotosamimanana, Head of the Mycobacteria Unit, Institut Pasteur de Madagascar.
Tuberculosis is a multi-factorial problem for which there will not be a simple science-based silver bullet. However, the genomics-based methods we have discussed here are a step forward in the road towards same-day diagnostics and transmission control, underpinned by open access data sharing to accelerate scientific progress. This work requires the input of many different researchers, clinicians, and public health workers from different backgrounds and disciplines, and without this network of collaborators, this simply would not be possible. Work on the genetic basis of drug resistance is ongoing in our lab—and across EMBL as part of the Infection Biology research theme—and we hope that as a society, by combining scientific progress with improvements in drug development, poverty alleviation, and access to medicines and diagnostics, we will make progress against this disease.
Source: Read Full Article