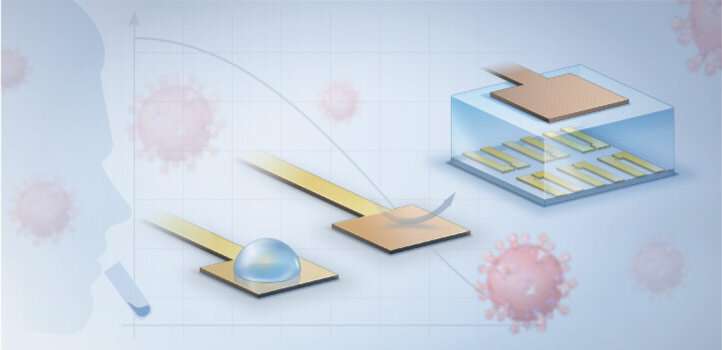
A new rapid coronavirus test developed by KAUST scientists can deliver highly accurate results in less than 15 minutes.
The diagnostic, which brings together electrochemical biosensors with engineered protein constructs, allows clinicians to quickly detect bits of the virus with a precision previously only possible with slower genetic techniques. The entire set-up can work at the point of patient care on unprocessed blood or saliva samples; no laborious sample preparation or centralized diagnostic laboratory is required.
“The combination of state-of-the-art bioelectronic hardware, materials science engineering and synthetic biology protein design really makes it possible to simplify and accelerate coronavirus testing,” says Raik Grünberg, a biochemist at KAUST who co-led the study.
Grünberg and his KAUST colleagues, including Sahika Inal and Stefan Arold, are now working with commercial partners to adapt their lab-scale prototype. They hope to create a bench-top, portable device that can be deployed to help contain the COVID-19 pandemic.
“This biosensor technology could be adapted to detect other pathogens and, as such, will have a major impact on controlling pandemics—today and in the future,” Inal says.
Coronavirus testing remains as important as ever. Despite increasing rates of vaccination in Saudi Arabia and around the world, global case numbers of COVID-19 remain at worryingly high levels, and public health officials need ways of rapidly identifying individuals who have contracted the disease so that they can limit virus transmission.
Current testing paradigms generally fall into two camps: either they detect viral RNA through genetic means, which can be slow and involves enzymatic amplification of trace molecular signals, or they capture viral proteins (known as antigens) in ways that are fast but not nearly as accurate.
The new KAUST technique now combines the speed of protein detection with the precision of genetic tests. “Even if there’s only a single virus particle in a sample, our platform will detect it,” says Keying Guo, a postdoc in Inal’s lab and co-author of the study with his KAUST colleagues Shofarul Wustoni and Anil Koklu.
The system starts with a virus-specific nanobody, a type of binding protein that can be designed to stick to fragments of different coronaviruses, including those responsible for COVID-19 and Middle East respiratory syndrome (MERS). The nanobody is tethered through a series of biochemical linkers to a thin layer of gold that, when an electric current is added, controls the flow of electricity through the semiconducting film it is connected to. The presence of any nanobody-bound viral proteins changes that flow, creating a signal that is amplified to measurable levels by a device known as an organic electrochemical transistor.
The researchers initially honed their test on human saliva and blood samples spiked with bits of protein from the coronaviruses that cause MERS and COVID-19. They then collaborated with doctors from KAUST Health in Thuwal and with scientists from King Faisal Specialist Hospital and Research Center in Riyadh to validate the assay on clinical specimens, both saliva and nasal swabs, collected from patients.
Source: Read Full Article